BIT team makes important progress in reduction of CO2 using microdroplets

Sponsored by

Recently, Professor Xie Jing's team from the School of Chemistry and Chemical Engineering at the Beijing Institute of Technology (BIT), in collaboration with Professor Zare's team from Stanford University, has made significant progress in the reduction of carbon dioxide (CO2) using microdroplets. The research results, titled "Molecular Mechanism for Converting Carbon Dioxide Surrounding Water Microdroplets Containing 1,2,3-Triazole to Formic Acid," have been published in the prestigious international publication Journal of the American Chemical Society. Professor Xie Jing and Professor Zare are the authors of the paper, with BIT being the first institution. This research project received funding from the National Natural Science Foundation of China and the BIT Teli Young Fellow Program, among other institutions.
In recent years, researchers have discovered that microscale liquid droplets can significantly accelerate chemical reactions and induce spontaneous partial redox reactions, all due to the unique physicochemical properties of microdroplets, such as extreme interfacial pH, partial solvation, or extremely high interfacial electric fields (~109 V/m). In 2022, Professor Zare's team used an ultrasonic nebulizer to spray a solution containing 1,2,3-triazole (Tz) into CO2 gas, achieving the reduction of CO2 to formic acid (HCOOH). This process does not require the involvement of metal catalysts and occurs under mild reaction conditions, thus offering an environmentally friendly potential solution for CO2 reduction. Experimental evidence suggests that this reaction occurs at the gas-liquid interface of microdroplets, but its microscopic mechanism has not been elucidated.
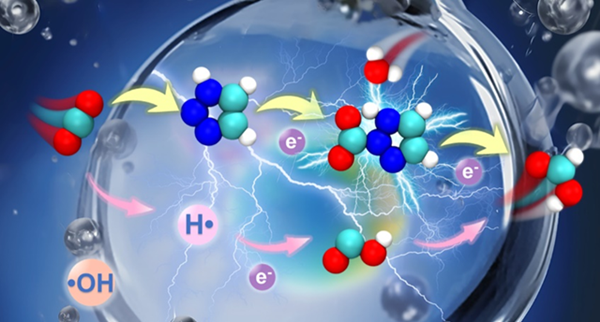
Figure 1. Schematic diagram of the CO2 reduction mechanism at the interface of water microdroplets containing 1,2,3-triazole. [Photo/english.bit.edu.cn]
Based on this, researchers conducted high-precision quantum chemical calculations and ab initio molecular dynamics simulations, combined with experimental validation, to reveal the microscopic mechanism by which Tz-containing water microdroplets convert CO2 into formic acid. Researchers first quantitatively calculated that the reduction of CO2 to formic acid by Tz-containing water microdroplets is a multi-step process. CO2 molecules are captured by Tz and form complexes on the interface of microdroplets, followed by reduction by one electron, and then intramolecular proton transfer occurs. Simultaneously, the complex decomposes into [Tz-H]- and COOH-, the latter spontaneously converting into formate anions. Additionally, the calculations indicate that the strong electric field and partial solvation at the microdroplet interface can promote intramolecular proton transfer, while interface acidity inhibits proton transfer. Moreover, the involvement of individual water molecules in the form of proton bridges can effectively lower the proton transfer barrier. Finally, researchers used ab initio molecular dynamics (AIMD) simulations to observe the "water-transporter" proton transfer mechanism at the gas-liquid interface (Figure 2). Combining the results of quantum calculations and dynamic simulations, researchers believe that the protons obtained by CO2 during the reduction process can come from water or Tz molecules.
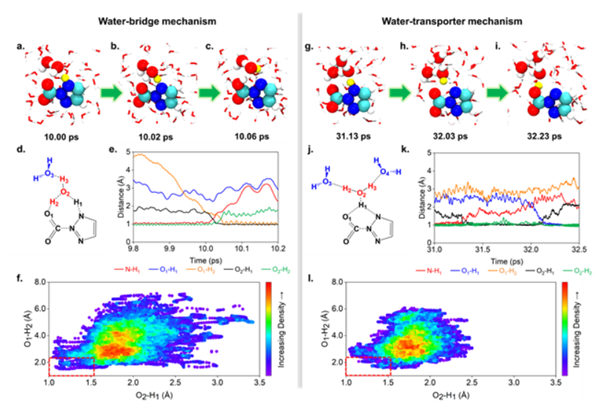
Figure 2. Proton transfer mechanism observed at the gas-liquid interface from ab initio molecular dynamics (AIMD) simulations. (a-c) "water-bridge" mechanism and (g-i) "water-transporter" mechanism, as well as (d-e) and (j-k) evolution of key bond lengths during the proton transfer process. (f-l) Configuration distribution of water molecules around the complex before proton transfer occurs. [Photo/english.bit.edu.cn]
This work reveals the microscopic mechanism of droplet experiments through quantitative calculations and dynamic simulations, elucidating how various factors of microdroplets interact and influence the kinetic process of CO2 conversion to formic acid in water microdroplets containing 1,2,3-triazole at the molecular level. This study holds profound significance for applying droplet chemistry to chemical synthesis and advancing the development of sustainable chemistry and green technologies.
Paper link: https://pubs.acs.org/doi/10.1021/jacs.4c00529
About the author
Xie Jing is a professor and doctoral supervisor at the School of Chemistry and Chemical Engineering of BIT. Her research interests include theoretical and computational chemistry, molecular reaction dynamics, microdroplet chemistry, and catalytic chemistry. The current research of her group mainly focuses on two aspects: 1. Microdroplet reaction kinetics simulation and theoretical research, including gas phase, micro-solvent phase, and gas-liquid interface chemical reaction kinetics simulation, exploring organic reactions, radical reactions, atmospheric reaction mechanisms, etc.; 2. Functional material design, including MOF/COF separation and catalytic material computation. She has been selected for the BIT Teli Young Fellow Program and the Young Elite Scientists Sponsorship Program by the Beijing Association for Science and Technology. She has undertaken projects for the National Natural Science Foundation of China, the Beijing Natural Science Foundation, and the Ministry of Science and Technology's foreign expert projects. So far, Xie has published more than 60 papers in academic journals such as Science, Nature, Acc. Chem. Res., J. Am. Chem. Soc., Angew. Chem. Int. Ed., Chem. Sci., Nature Chem., and J. Phys. Chem. Lett.