A new approach to stabilise perovskite solar cells without lead

Sponsored by

Sponsored by

Solar cells made from perovskite, a material that is able to harvest sunlight and convert it to electricity, hold great potential as a replacement for silicon solar cells.
Despite superior performance, efficiency and being cheaper to manufacture, perovskite solar cells have not been commercially manufactured for the consumer market.
Perovskites decompose when they react with moisture and oxygen or when they are exposed to light, heat, or used for an extended time, resulting in concerns that the small amount of lead, a toxic heavy metal, present in perovskite solar cells could pollute the environment when the solar cell is damaged or discarded.
The lead comes from both the perovskite material and a compound used to make a component of the perovskite solar cell, known as the capping layer.
Now, research by scientists from Nanyang Technological University, Singapore (NTU Singapore) and the Institute of Materials Research and Engineering (IMRE) at the Agency for Science, Technology and Research (A*STAR) in Singapore has provided the possibility of capping materials based on non-toxic metals being used in the manufacture of perovskite solar cells.
Their study, published in Nature Energy in February 2023 and led by Prof Sum Tze Chien, Director of the Institute of Advanced Studies at NTU and Associate Dean (Research) of NTU’s College of Science, and Prof Lam Yeng Ming, Chair of NTU’s School of Materials Science and Engineering, could take perovskite solar cells one step closer to the market.
Stabilising perovskite solar cells without lead
Perovskite solar cells are made of several layers of materials, including a perovskite layer that harvests light and a capping layer. The capping layer is coated onto the perovskite layer to protect the solar cell from environmental stresses such as heat and moisture and to boost the performance of the cell.
To ensure that the capping layer is compatible with the underlying perovskite layer, researchers typically use an approach called the half precursor (HP) method to fabricate the capping layer. One of the precursor chemicals is first deposited on top of the perovskite layer which provides the other precursor. Through a process known as cation exchange reaction, the deposited precursor then reacts with lead ions present in the perovskite layer beneath to form a lead-based chemical compound that makes up the capping layer.
As a result of the HP method, lead is also present in the protective capping layer. A method that enables non-toxic metals to be used in the capping layer would be a game-changer for perovskite solar cells.
To make perovskite solar cells more environmentally friendly, the NTU scientists devised an approach, known as a full precursor (FP) solution method, to synthesise a capping layer that does not contain lead.
Using the FP method, the scientists coated perovskites with solutions containing metal halide salts – compounds made of a metal and elements that include chlorine, fluorine and iodine – and phenethylammonium iodide (PEAI) that is commonly applied to perovskites to improve the performance of perovskite solar cells. PEAI contains ammonium, a positively-charged ion that contains nitrogen and hydrogen, which is vital for the chemical reaction.
Zinc-based compound leads the way
The researchers found that a zinc-based compound PEA2ZnX4 synthesised using the method was the most effective capping material among the other materials tested.
Unlike the HP method, there is no need to draw lead ions up from the underlying perovskite layer to form this protective capping layer when the FP method is used. This paves the way for the use of non-toxic metals in the capping layer.
According to the researchers, the FP process is also more effective than the HP method at fabricating the capping layer as the chemicals in the solution are able to react with each other directly on the surface of the perovskite.
To fabricate a solar cell capped with PEA2ZnX4, the researchers first dissolved zinc halide salts and PEAI in a solvent called acetonitrile, commonly used for industrial applications. They then deposited the solution onto a rapidly-spinning perovskite layer attached to an electrically conductive glass substrate to form a thin and uniform layer – a process known as spin-coating. The coated perovskite was heated at 100 degrees Celsius for 10 minutes to bind the capping layer to the surface of the perovskite. The researchers then used a process called vacuum evaporation, in which a material is heated in a vacuum chamber to produce a vapour which is deposited on the coated perovskite, to synthesise the other layers of the perovskite solar cell.
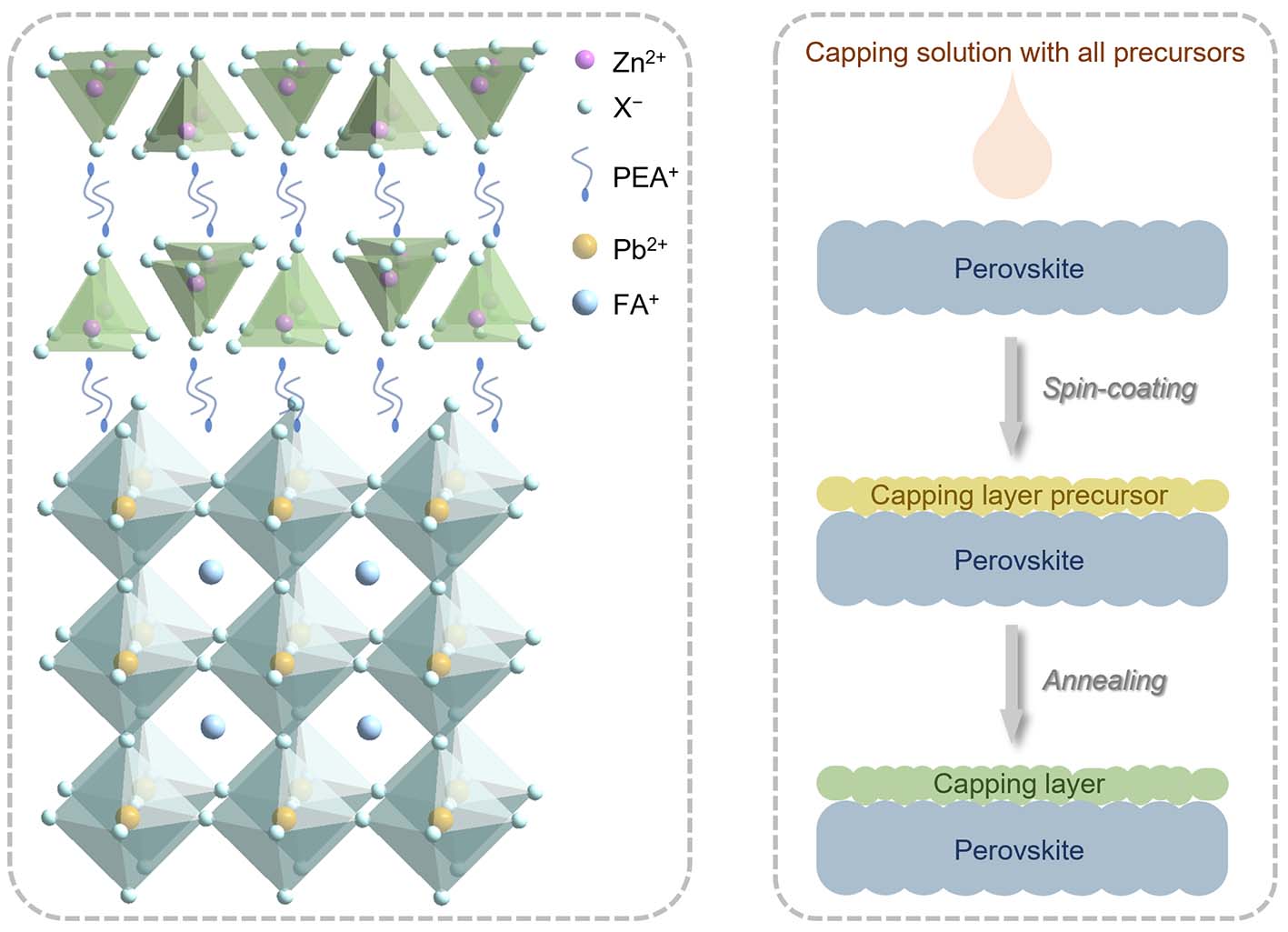
(Left) The molecular structure of perovskite (blue) with the zinc-based capping layer (green). (Right) The FP process that the researchers used to coat the zinc-based capping layer onto the perovskite layer. Credit: NTU Singapore.

To fabricate the zinc-based capping layer, the researchers dissolved the chemicals and coated the solution onto the perovskite layer in a glovebox. The glovebox protects the perovskite from oxygen and moisture in the environment before it is coated. Credit: NTU Singapore.

The researchers used a vacuum evaporation system to synthesise the remaining layers of the perovskite solar cell, a method commonly used for the fabrication of perovskite solar cells. Credit: NTU Singapore.
Using the FP method, the scientists created a 1 inch by 1 inch prototype solar cell capped with the zinc-based compound.
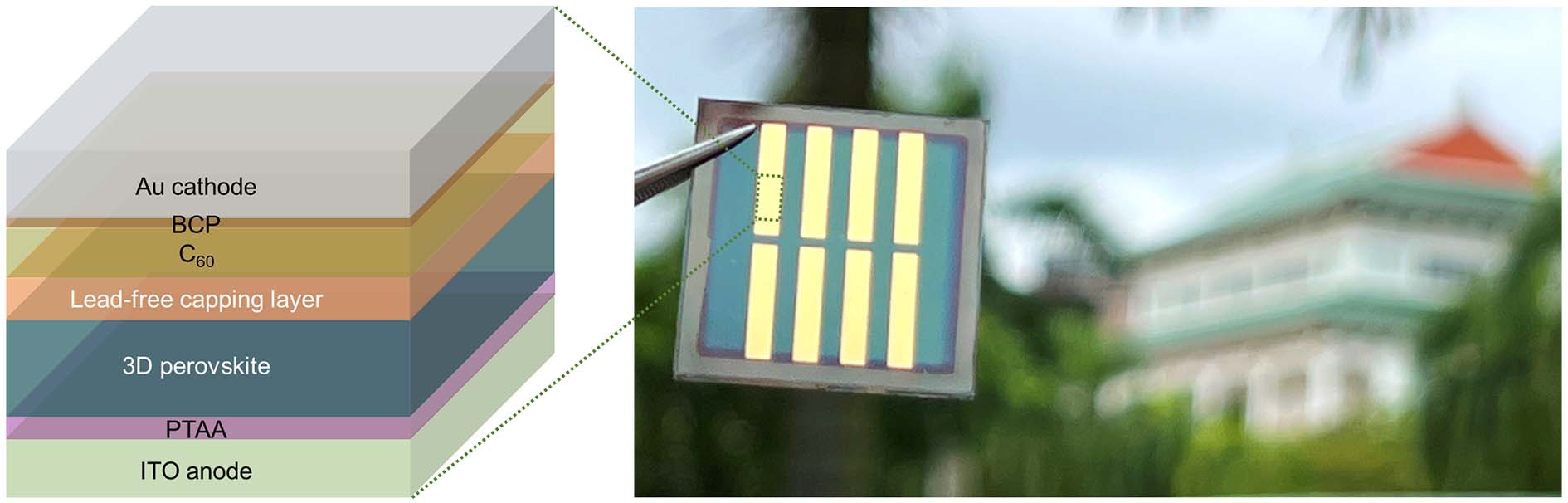
(Left) A diagram showing the different layers of the perovskite solar cell capped with the zinc-based capping material fabricated by the researchers. (Right) The dotted green rectangle indicates the active region of the perovskite solar cell that captures sunlight and converts it to electricity. Credit: NTU Singapore.
The scientists examined the zinc-based capping layer using electron microscopy and spectroscopy and found that it did not affect the electrical properties of the underlying perovskite layer. The capping layer also helped to cover defects on the surface of the perovskite layer and improved its light-harvesting capabilities.
The researchers say that their method contributes to efforts to make perovskite solar cells more environmentally friendly. For example, the method can be used together with lead-free perovskite materials that are currently being explored for the development of solar cells that would not release toxic metals when discarded.
The method also opens the possibility of engineering the composition of the capping layer to enhance the performance of perovskite solar cells.
“One of the biggest drawbacks of using perovskite solar cells is their impact on the environment. By enabling zinc and other non-toxic metals to be used in the capping layer, our innovation potentially solves a major obstacle that prevents the widespread use of perovskite solar cells,” said Dr Ye Senyun, research fellow from NTU’s School of Physical and Mathematical Sciences, one of the lead researchers of the study.
Co-lead author Dr Rao Haixia, research fellow from NTU’s School of Materials Science and Engineering, said: “As our method does not require extracting lead ions from the perovskite, this enables the possibility of using a wide range of materials to boost the stability and efficiency of perovskite solar cells.”
The researchers found that the fabricated solar cell was as effective at converting sunlight to energy as conventional perovskite solar cells. In experiments with light that simulated sunlight, the solar cell could convert 24.1 per cent of the light captured to electricity, close to the highest efficiency achieved by perovskite solar cells so far. In comparison, the highest certified light conversion efficiency of silicon solar cells is 25.2 per cent.
“The capping layer is a vital component of a perovskite solar cell. This new approach to fashion the capping layer is a major milestone in designing perovskite solar cells with enhanced properties,” said Prof Michael Grätzel, Director of the Laboratory of Photonics and Interfaces at the École Polytechnique Fédérale de Lausanne in Switzerland, who was not involved in the study.
The prototype also demonstrated good reproducibility, with an average light conversion rate of almost 23 per cent over 103 cells tested. It had a long lifespan, maintaining more than 90 per cent of its ability to convert light into electricity for more than 1,000 hours of operation at full capacity.
The performance of the device was also more stable at high temperatures compared to devices without a capping layer.
“By expanding the library of materials that can be used, our findings unlock new opportunities for developing superior materials for the capping layer, for more efficient and stable perovskite solar cells,” said Prof Sum. “This lead-free protective capping method can also be extended to other applications like perovskite light emitting devices, lasers and detectors.”
Prof Lam added, “As fossil fuels are depleting rapidly, we need to harness renewable energy resources such as solar energy much better. Eco-friendly and stable perovskite solar cells could be the answer and this innovation can potentially propel a wider scale adoption of perovskite solar cells for solar energy harvesting.”
The scientists are working on scaling up the method to fabricate full-sized solar cells. They are also in the process of filing a patent with NTUitive, NTU Singapore’s innovation and enterprise company.
Details of the study can be found in “Expanding the low-dimensional interface engineering toolbox for efficient perovskite solar cells” in Nature Energy (2023).