Stopping Alzheimer's in its tracks
Sponsored by

Sponsored by

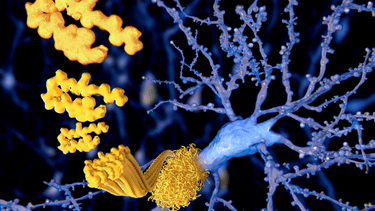
Its most common form, Alzheimer’s disease, occurs when two proteins, amyloid-ß and tau, accumulate in the brain because our neurons make too much – or clear too little – of them.
As the clumps of amyloid-ß (plaques) and tau (tangles) increase in number, they become toxic and create ‘roadblocks’ – killing neurons, shrinking the brain, and preventing memories from being formed. Hence the inability to carry out previously simple daily activities – including basic functions such as breathing.
Researchers at UQ’s Queensland Brain Institute (QBI) have been studying these ‘roadblocks’ for more than a decade and believe they are now on track to eliminating them – and possibly Alzheimer’s disease itself.

MILD – Amyloid-ß (yellow) starts to accumulate near neurons; tau starts to accumulate inside neurons. Image: Richard Campbell

MODERATE: Amyloid-ß plaques increase. Tau (black) begins to deposit inside the neuron, causing damage. Image: Richard Campbell
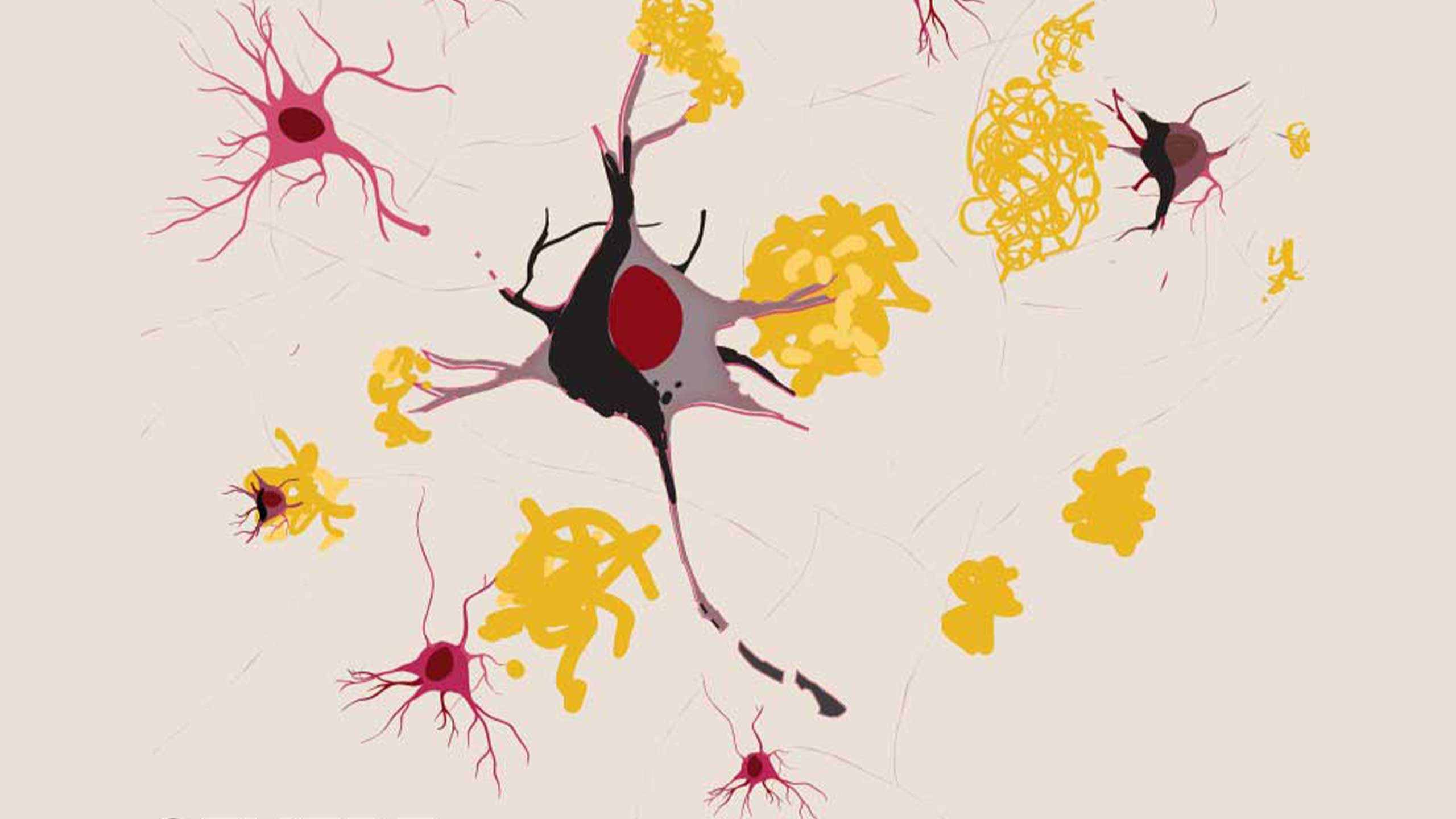
SEVERE: Death of neurons occurs, severing connections with other neurons. Image: Richard Campbell
Ultrasound is key
According to Professor Jürgen Götz, Director of the Clem Jones Centre for Ageing Dementia Research (CJCADR) – currently celebrating its 10th anniversary – ultrasound is the key.
“Following our pioneering discovery, published in 2015, that scanning ultrasound can remove amyloid-ß build-up in mouse brains, we are hopeful that it could also work for people,” Professor Götz said.
“Our research demonstrated that amyloid-ß was removed and memory restored without the use of any therapeutic agent, and the scanning treatment did not induce any apparent damage to the mouse brain.
“The scanning ultrasound activated resident microglial cells that took up amyloid-ß into their lysosomes, suggesting that repeated scanning ultrasound may be a non-invasive method with potential for treating Alzheimer’s disease.
“This work has enormous potential for reversing one of the most pressing health issues in a rapidly ageing society.”
So, what spurred the researchers to investigate ultrasound rather than traditional pharmaceutical intervention?
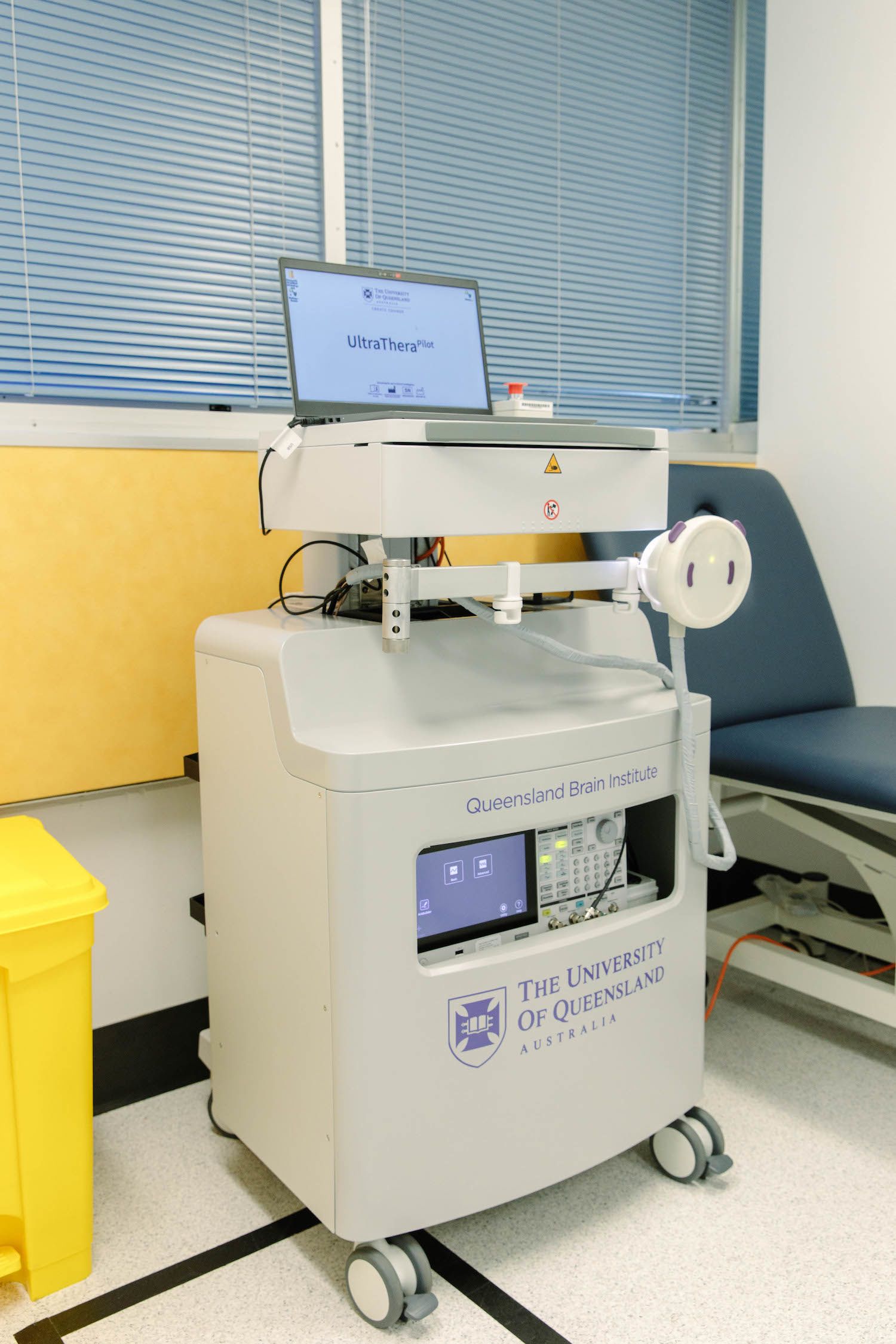
The UltraThera pilot ultrasound machine to be used in clinical trials. Image: QBI
Overcoming the blood-brain barrier
Ideally, drugs would be used to remove the toxic build-up of plaque and tau to reverse cognitive damage, but their delivery is hampered by the blood-brain barrier, which acts as a strong shield stopping drugs from penetrating to where they are needed.
A way to open this barrier needed to be found.
Serendipitously, Professor Götz was attending an international medical conference and noticed a poster where someone had used ultrasound for the delivery of a drug into the brain.
“On my flight back, I recalled the poster and thought ‘why not use ultrasound in an Alzheimer mouse with amyloid plaques to open the blood-brain barrier and thereby flush out the amyloid?’
“So, we bought an ultrasound machine for small animals (the last of a small series of 20+ machines) and did the experiments and, surprisingly, found that ultrasound, by opening the blood-brain barrier, did not flush out the amyloid into the blood stream for clearance.
"Instead, mechanisms in the brain were activated, which led to amyloid clearance by the brain’s own microglia.”
To achieve this, his team optimised the ultrasound and other parameters and also established a scanning approach that allowed them – assisted by microbubbles injected into the bloodstream – to temporarily open the blood-brain barrier. They also showed that with this methodology, antibodies – and by extension other drugs – could be effectively delivered to reach both amyloid plaques and tau tangles and ultimately clear them, restoring cognitive function.
The larger team of medical engineers and biologists then used this insight to build a device suitable for use in humans.
They later found that ultrasound alone could be just as effective in restoring cognitive function even in the absence of removing amyloid plaques.
But would it work on humans?
The research team is continuing to work towards a small safety trial in Brisbane that will be the first step in the next part of the journey.
Partnering for success
Professor Götz’s team has made it a point to consistently collaborate with stakeholders and prove the worthiness of the research for financial support – a vital task given that Alzheimer’s disease is the second leading cause of death in Australia.
QBI Director Professor Pankaj Sah agrees.
“Since its inception, CJCADR has championed dementia research within the neuroscience community,” he said.
“These 10 years of research would not have been possible without the philanthropic support of the Clem Jones Foundation, our many donors, and the combined support of state and federal governments.”
From its humble beginnings with a handful of scientists, today CJCADR within QBI consists of 10 labs with more than 90 researchers dedicated to understanding the cause of dementia and to designing new treatments and preventative approaches.
Clem Jones Group Chair David Muir and QBI's inaugural Director, Emeritus Professor Perry Bartlett, were instrumental in its early origins. It was their shared vision to create a centre for dementia research that would attract new talent to Queensland to increase understanding of brain ageing and dementia.
“Clem recognised that scientific research is both costly and a long-term process with few genuine ‘overnight' successes,” Mr Muir said.
“Meaningful breakthroughs are more often the result of hard work by researchers and the commitments made by their backers over many years.
“Clem was exposed on numerous fronts to the impact a range of dementias can have on individuals and those around them.
“And while Clem was no different to many other Australians in that regard, he knew he could help make a difference by supporting dementia research.”
Neuroscience discoveries are often a long time in the making and require enormous investments in time and resources, but ultimately, they have the potential to improve millions of lives. Professor Götz and his team are very happy to ‘hasten slowly’ and get it right to ensure this insidious disease can be stopped quickly in its tracks.
Looking ahead to the next decade, the talented team at CJCADR is optimistic about the opportunities for their research to contribute important knowledge and improve the outcomes for people living with dementia.
Key milestones
Pre-2015:
- Research begins at the Clem Jones Centre for Ageing Dementia Research, which leads to the invention that ultrasound combined with small gas-filled bubbles opens the blood-brain barrier in mice, resulting in clearance of amyloid plaque build-up – a hallmark of Alzheimer’s disease – and improvement in memory and cognition.
2015:
- The research on non-invasive ultrasound technology that could be used to treat Alzheimer’s disease and restore memory in mouse models is published together with the mechanism of action in which immune cells in the brain are activated by the ultrasound-microbubble blood-brain barrier opening.
- A first-generation test platform is designed and built to enable researchers to simulate, visualise and characterise the effects of the ultrasound through skull bone.
- Large animal principle study commences.
2016:
- A specialist medical device engineering firm is engaged to begin work alongside the research team on early iterations of the technology.
- First demonstration of blood-brain barrier opening with focused ultrasound in sheep takes place, showing that ultrasound can indeed pass through skulls of a similar thickness to humans.
2017:
- A second-generation test platform is designed, built and commissioned. The liquid-filled structure guides researchers’ selection of the frequency and pulse sequence of ultrasound to be used in their studies, including how passing through the bones of the skull would alter the beams’ path.
- The first pre-clinical research ‘probe’ is designed to direct and deliver ultrasound waves to the brain.
- The sheep study demonstrates for the first time the (deeper) cortical blood brain barrier opening with focused ultrasound.
2018:
- Research ‘probe’ is built and verified in the second-generation test platform.
- Animals treated with focused ultrasound using pre-clinical research probe are shown to recover satisfactorily.
2019:
- Animal safety study is conducted with pre-clinical research probe and MRI analysis.
- UltraThera prototype system is built and bench-tested, then successfully demonstrated in a multiple treatment animal trial.
- Protocols are developed for first ‘in-human’ safety trials.
2020:
- Working with industrial design partner Tiller Design, phase 1 and 2 UltraThera prototype system fabrication commences.
- COVID-19 halts repeat dose trial and planning begins for scanning ultrasound-only clinical trial.
2021:
- Low-intensity ultrasound without microbubbles is shown to restore memory in aged mice; the published results provide the impetus for using ultrasound alone without blood-brain barrier opening in the first in human clinical trial.
- A large animal pre-clinical study of scanning ultrasound in Alzheimer’s disease is completed using UltraThera pilot system.
2022
- Research team intensify efforts to prepare for the first clinical trial of the UltraThera system.

Professor Jürgen Götz. Image: QBI
Patents and publications
2014:
- Scanning Ultrasound (SUS) provisional patent is filed.
2015:
2016:
- Ultrasound treatment of neurological diseases — current and emerging applications
- Scanning Ultrasound (SUS) Causes No Changes to Neuronal Excitability and Prevents Age-Related Reductions in Hippocampal CA1 Dendritic Structure in Wild-Type Mice
2017:
- Combined effects of scanning ultrasound and a tau-specific single chain antibody in a tau transgenic mouse model
- Ultrasound as a treatment modality for neurological diseases
2018:
- SUS patent is published.
- Safety and efficacy of scanning ultrasound treatment of aged APP23 mice
- Establishing sheep as an experimental species to validate ultrasound-mediated blood-brain barrier opening for potential therapeutic interventions
- Modeling ultrasound propagation through material of increasing geometrical complexity
- Multimodal analysis of aged wild-type mice exposed to repeated scanning ultrasound treatments demonstrates long-term safety
2019:
- Repeated ultrasound treatment of tau transgenic mice clears neuronal tau by autophagy and improves behavioral functions
- Ultrasound-mediated blood-brain barrier opening enhances delivery of therapeutically relevant formats of a tau-specific antibody
- Scanning ultrasound in the absence of blood-brain barrier opening is not sufficient to clear β-amyloid plaques in the APP23 mouse model of Alzheimer's disease
2020:
- The blood-brain barrier: Physiology and strategies for drug delivery
- Altered Brain Endothelial Cell Phenotype from a Familial Alzheimer Mutation and Its Potential Implications for Amyloid Clearance and Drug Delivery
- Delivery of antibodies into the brain using focused scanning ultrasound
2021:
- SUS + Tau Antibodies patent is published.
- Therapeutic Ultrasound as a Treatment Modality for Physiological and Pathological Ageing Including Alzheimer’s Disease
- Low-intensity ultrasound restores long-term potentiation and memory in senescent mice through pleiotropic mechanisms including NMDAR signaling
- A comparative study of the effects of Aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer disease
2022:
- SUS + Amyloid-beta Antibodies, and SUS Cognition patents are published.
- US grants SUS patent.
- Transcriptional signature in microglia isolated from an Alzheimer's disease mouse model treated with scanning ultrasound
- Ultrasound-Mediated Bioeffects in Senescent Mice and Alzheimer’s Mouse Models
- Opportunities and challenges in delivering biologics for Alzheimer’s disease by low-intensity ultrasound
- Ultrasound-mediated delivery of novel tau-specific monoclonal antibody enhances brain uptake but not therapeutic efficacy